FDA’s Detection of Banned Antibiotics in Indonesian Shrimp Continues in 2026
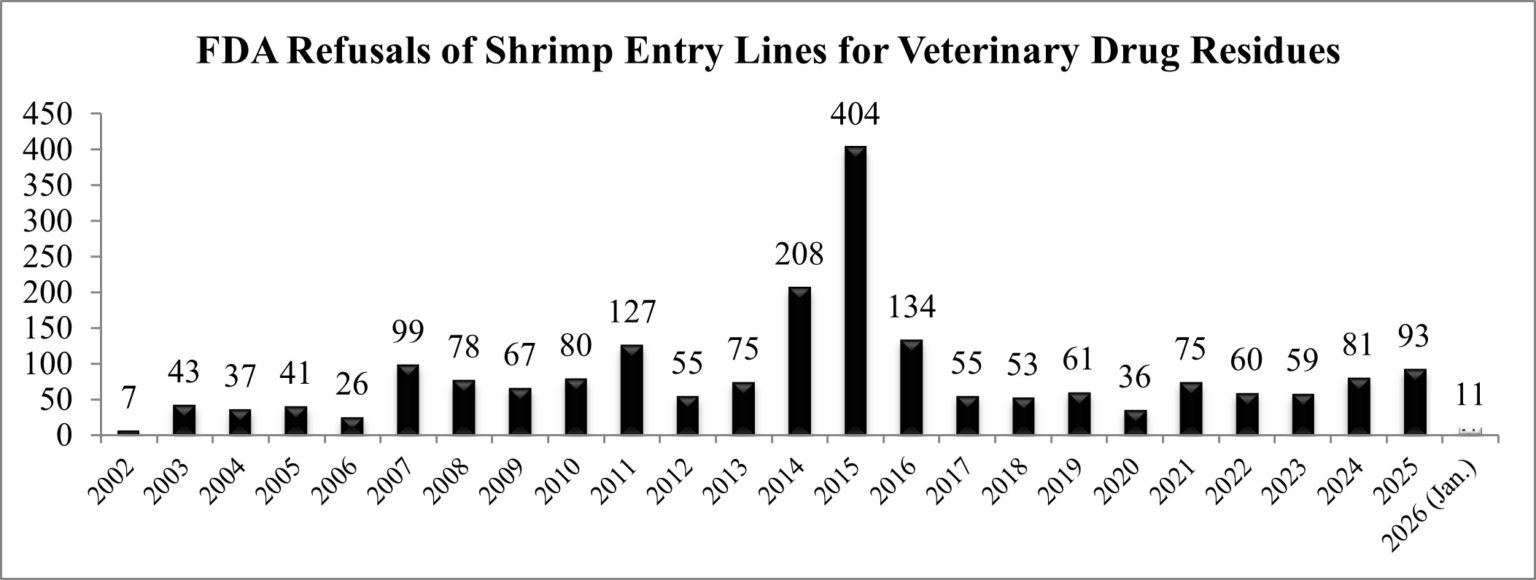
As shown in the chart below, the FDA’s reporting of shrimp entry line refusals for reasons related to banned antibiotics continues the trend of increasing rejections over the last three years.
As explained in more detail below, seven of the entry line refusals in January were of shrimp from Indonesia, continuing the FDA’s response to the Indonesian shrimp aquaculture’s industry adoption of banned antibiotics. To put the seven entry line refusals of Indonesian shrimp in January 2026 in context, over the nine-year period spanning from 2016 through 2024, the FDA refused a grand total of eight Indonesian shrimp entry lines for reasons related to veterinary drug residues.
The eleven (11) reported entry lines of shrimp refused for veterinary drug residues in January were attributed to shipments from four different exporters in Hong Kong, Indonesia, and Vietnam. As noted below, three of these four exporters are Best Aquaculture Practices (BAP)-certified shrimp processors:
PT. Tamron Akuatik Produk Industri (Indonesia), a company that currently operates under a four-star BAP certification for its processing plant (P10704) and that was added to Import Alert 16-129 (“Detention Without Physical Examination of Seafood Products Due to Nitrofurans”) on August 28, 2025 for the presence of nitrofurans in its shrimp, had one entry line refused for shrimp contaminated with nitrofurans and veterinary drug residues by the Division of Southeast Imports on January 7, 2026; one entry line refused for breaded shrimp contaminated with nitrofurans and veterinary drug residues by the Division of Southwest Imports on January 9, 2026; one entry line refused for breaded shrimp contaminated with nitrofurans and veterinary drug residues by the Division of Southeast Imports on January 15, 2026; one entry line refused for breaded shrimp contaminated with nitrofurans by the Division of Northeast Imports on January 20, 2026; one entry line refused for breaded shrimp contaminated with nitrofurans and veterinary drug residues by the Division of Northern Border Imports on January 23, 2026; one entry line refused for breaded shrimp contaminated with nitrofurans and veterinary drug residues by the Division of Northeast Imports on January 27, 2026; and one entry line refused for shrimp contaminated with nitrofurans and veterinary drug residues by the Division of Southeast Imports on January 28, 2026;
Gallant Dachan Seafood Co., Ltd. (Vietnam), a company that currently operates under a three-star processor BAP certification (P10436) and is not currently listed on Import Alert 16-124 (“Detention Without Physical Examination of Aquaculture Seafood Products Due to Unapproved Drugs”), Import Alert 16-127 (“Detention Without Physical Examination of All Seafood Due to Chloramphenicol”), or Import Alert 16-129 (“Detention Without Physical Examination of Seafood Products Due to Nitrofurans”), had two entry lines refused for breaded shrimp contaminated with nitrofurans by the Division of Southeast Imports on January 2, 2026;
Seavina Joint Stock Company (Vietnam), a company that currently operates under a four-star processor BAP certification (P10387) and is not currently listed on Import Alert 16-124 (“Detention Without Physical Examination of Aquaculture Seafood Products Due to Unapproved Drugs”), Import Alert 16-127 (“Detention Without Physical Examination of All Seafood Due to Chloramphenicol”), or Import Alert 16-129 (“Detention Without Physical Examination of Seafood Products Due to Nitrofurans”), had one entry line refused for breaded shrimp contaminated with nitrofurans by the Division of Southeast Imports on January 21, 2026; and
Hoptai Marine Products Company Limited (China), a company that is not currently included on the “green” list for Import Alert 16-131 (“Detention Without Physical Examination of Aquacultured Shrimp, Dace, and Eel from China and Hong Kong SAR – Presence of New Animal Drugs and/or Unsafe Food Additives”), had one entry line refused for shrimp contaminated with veterinary drug residues by the Division of Northeast Imports on January 30, 2026.
In addition to these rejects, the FDA’s Division of West Coast Imports reported refusing an entry line of Vietnamese shrimp manufactured by Fimex VN for “ADDED BULK” on January 6th after sampling the shipment.
Soure: shrimpalliance
AQUA MINA CO., LTD
– Address: 685 Le Duc Anh Street, Quarter 39, Binh Hung Hoa Ward, Ho Chi Minh City
– Phone: 1800 6071 (Toll-free hotline)
– Email: sales@aquamina.com.vn or oversea@aquamina.com.vn
– Aqua Mina’s Official Distributor in Japan: REX INDUSTRIES CO., LTD
– Address: 1-9-3 Hishiya-Higashi, Higashi-Osaka 578-0948, JAPAN
– Email: kimakubo@rexind.co.jp
– Phone: +81-(0)72-961-9893
– Website: www.rexind.co.jp/e/

WE WORK FOR THE SUCCESS
Ngày đăng : 22/02/2026
1014 View
Other Articles
5 groups of people who should not eat shrimp, no matter how much they love it, to avoid nviting illness into the body
The United States significantly reduces anti-dumping duties on Vietnamese frozen warmwater shrimp
Shrimp exports this year are estimated to reach 4 billion USD
New technology helps dried seafood rehydrate like fresh
Georgia Senate Resoundingly Passes Shrimp Transparency Bill
Situation of Whiteleg Shrimp Farming in Southeast Asian Countries
Indian shrimp pivot to the EU, increasing competitive pressure on Vietnam
Indoor shrimp farming in Europe: Investment challenges and the race to find a viable model
Shrimp production surged in the first month of the year, with exports benefiting from strong demand during the Lunar New Year holiday
Quang Ninh Accelerates Digital Transformation in Shrimp Farming, Rising to Lead Northern Vietnam
Lucky money is not just about cash — it’s Aqua Mina’s wish for a worry-free farming season for our valued customers
Việt Nam's top 10 seafood exporters command nearly one-fifth of industry revenue



















.jpg)